|
ABSTRACT Hydroxypropyl methylcellulose (HPMC) is employed for a wide variety of pharmaceutical and food preparations. Its applications as viscolizing agent (thickening agent), coating polymer, bioadhesive, in solid dispersion to enhance solubility, binder in the process of granulation and in modified release formulations have been well documented. One other notable use is in the production of capsule shells, replacing the animal derived gelatin in conventional two-piece capsules. The aim of this review is to systemically survey published literature on the HPMC use in capsule shells and resolve questions regarding their suitability as a replacement for hard gelatin capsules. Future refinements in the production and filling of HPMC capsule shells and improvement in their in vivo/in vitro dissolution would ensure their superiority over hard gelatin capsules.
Hydroxypropyl methylcellulose (HPMC), now commonly known as hypromellose, is produced by synthetic modification of the naturally occurring polymer cellulose and is considered safe for normal consumption in humans. The material have been used and experimented as viscolizing agent i.e.thickening agent, in controlled release systems and as a coating polymer, as a bioadhesive, in solid dispersion to enhance drug solubility and as a binde. The material is described as a white to slightly offwhite powder or granules, hygroscopic after drying, practically insoluble in hot water, in acetone, in dehydrated ethanol and in chloroform, but dissolves in cold water giving a colloidal solution owing to the reversible thermal gelation property. HPMC is available in different substitution type with limits on methoxy and hydroxypropoxy groups. These groups influence many of the HPMC properties such as gelation temperature, viscosity, flexibility and hydration.
In addition to the listed excipient categories, HPMC polymer is now used as shell material for capsules. The origin of the word capsule comes from the Latin capsula, which means a small box. Pharmaceutically, capsules are either hard (two-piece) or soft (one-piece) and are used to encapsulate pharmaceutical formulations. The two-piece capsule is made of a cap-piece that slips cylindrical object. Capsules may offer better solid dosage form to tablets for drugs with low compressibility, slow dissolution and bitter tasting. They are also used in clinical studies for blinding purpose.
HPMC capsules may offer attractive alternative to gelatin capsules because of its vegetable source. The crosslinking of gelatin and drug incompatibilities and the strict regulations regarding the use of animal derived gelatin requiring the absence of bovine spongiform encephalopathy (BSE)/ transmissible spongiform encephalopathy (TSE) have encouraged the search for gelatin replacement. Religious, cultural and personal issues may affect patients’ preference towards the medications presented in capsule dosage forms.
Mechanical Strength
In a test examining the effect of humidity on the mechanical properties of both HPMC and gelatin capsules, it was found that both types of capsules softened, especially above 60% of relative humidity, with gelatin capsules exhibiting in general higher stiffness and hardness values compared to the HPMC capsules. In another study it was found that at ambient conditions, capsules made from gelatin were harder and stronger but less elastic compared with HPMC counterparts.
Effects of Ambient Conditions
The use of capsules as means for rapid disintegration in the oral cavity was experimented. One approach was to cause the capsules to become brittle in order to brake rapidly in the oral cavity by subjecting them to low humidity. While this approach was successful for hard gelatin capsules, HPMC capsules remained flexible, even at low moisture content. Short term stability studies (heating at different temperature for 24 hrs) found that the HPMC shell exhibits a significantly better short term stability at high temperature than hard gelatin capsules on visual test, disintegration and dissolution, as well as mechanical property assessment. When they were stored at different relative humidity (RH), the HPMC capsules exhibited lower moisture contents compared to gelatin capsules (e.g. 6% and 14% respectively at 50% RH) that have shown to be more hygroscopic. Based on the previous study, the specifications for moisture content are 2–7% for the HPMC shell corresponding to RH 10–60% and 13–16% for gelatin capsules corresponding to storage at RH 35– 65%. Preliminary results from the effect of irradiation (beta or gamma) on both HPMC and gelatin capsules in air indicated their suitability for ionizing radiation sterilization.
IN VITRO Disintegration and Dissolution
Because the USP only mentions the testing of gelatin capsules, Donauer and Löbenberg have called in a min review the USP to specify how to carry out the disintegration test with HPMC capsules. That is because the dissolution behaviors of HPMC and gelatin capsules have to be different in dissolution media. Moreover, HPMC capsules are not all the same as they may or may not contain a gelling agent and the gelling agents used are not all the same.
The shell dissolution properties of ordinary gelatin hard capsules, gelatin/PEG capsules and HPMCcarr capsules were compared independent of their capsule content . Different dissolution media and storage conditions were used. The capsule shells disintegration/dissolution time was determined as the time for enough parts of the suspended capsule to dissolve, permitting steel ball bearing filled into the capsule to fall free. Capsules were placed in media of different temperature (between 10º and 55º C) in order to simulate taking the capsules with cold, warm or hot drinks. The dissolution media in the glass beaker at different temperatures were brought back to 37º C with the controlled temperature of the surrounding water bath. Gelatin and gelatin/PEG capsules disintegrated rapidly and faster than the HPMCcarr capsules in the different media following storage at different conditions when tested at temperature ≥ 37º C. This delay in the HPMC capsule disintegration was especially notable in mixed phosphate buffer of pH 6.8. The delay at pH 6.8 is inherent for the HPMC shells. In water at 37 º C following storage at ambient room conditions (19±1 °C, 35-40% relative humidity of the air) HPMCcarr capsules disintegrated in approximately 4 minutes whereas gelatin and gelatin/PEG capsules disintegrated in approximately 1 minute. Gelatin capsules dissolution times are dependent upon temperature and generally do not dissolve at temperatures below 30 °C, however, their dissolution was rapid as temperature increased from 30° to 55°C suggesting that gelatin capsules are better be taken with warm water.
The influence of the composition of test fluids on dissolution from HPMCcarr capsules in comparison to the hard gelatin capsule was studied. The results were in agreement with another study showing significant retarding effect of potassium and/or calcium ions in the dissolution medium, while the effect of pH was minimal on the acetaminophen (BCS class III) dissolution. Similar effects of dissolution media were also documented when studying the effects of dissolution medium, capsule grade and capsule size on the in vitro rupture time of the capsule shells. The results further indicated that the capsules used whether pharmaceutical HPMCcarr capsules, nutritional HPMCcarr capsules or gelatin capsules all rupture in different times in vitro with gelatin capsules being the fastest. Stein and Bindra who used HPMC capsules for their formulations found that in an acidic pH (0.1 N HCl), the dissolution of the capsules formulations were retarded in comparison to hard gelatin capsules at earlier times and therefore delaying the time of complete drug dissolution.
Size 0 hard HPMC and gelatin capsules were tested for ibuprofen (BCS class II) release in tribasic sodium phosphate buffer (pH 7.2, 900 ml at 37 °C) with formulation containing release modifiers (powdered HPMCs grades as diluents). As the medium contained no potassium (apparently to prevent its influence on the dissolution from HPMC capsules), both types of capsules showed similar dissolution profiles. However, it is apparent that such formulations influence their own release, irrespective of the capsule shell rupture time, therefore not sharp indicative of the capsule rupture time in the dissolution medium.
Honkanen showed that when ibuprofen formulation in HPMCcarr capsules tested for drug release in a neutral potassium phosphate buffer, it was incomplete and highly variable compared with the gelatin capsules and attributed this to the presence of potassium ions (K+) in the dissolution medium that causes the capsule shell to form a membrane around the filling. Because the gut concentration of potassium is low, she justified the change of dissolution medium to neutral tribasic sodium phosphate which resulted in complete and less variable drug release. In this medium 100% of the drug was released for both types of capsule within 15-20 minutes, however, there was a lag time of approximately 4 minutes before the drug release from HPMCcarr capsules, unlike gelatin capsules in which the release was immediate.
Similar results were obtained when testing the dissolution of HPMCgell capsule shells that are filled with ibuprofen in comparison to hard gelatin capsules at pH 7.2 using potassium phosphate and TRIS buffers. It was found that the presence of K+ cations retards HPMCgell capsule opening with the drug dissolution approaching 60% after 60 minutes compared to approximately 95% at 10 minutes for hard gelatin capsules. It was also reported that for acetaminophen, the release delay was lessened when sodium ions were present instead of potassium in phosphate buffer at pH 7.2 or in acetate buffer at pH 4.5 and that the HPMCgell capsules failed to rupture with very little drug release when the medium was acidic (0.1 N HCl, pH=1.2). The authors explained the hindrance of the HPMCgell capsule dissolution in potassium phosphate buffer as due to the monovalent cations binding to the surface of individual helices of gellan, thus lowering their charge-density and reducing the electrostatic barrier to aggregation and hence solubility is reduced. They proposed that sodium ions do not efficiently bind as potassium ions and therefore disruption will be faster.
They also explained that unlike the sulfate groups in carrageenan gelling system, the carboxyl groups of gellan gum, have a much higher pKa resulting in uncharged (-COOH) form at low pH. This elimination of electrostatic repulsion between helices makes gellan less soluble at pH 1.2. HPMC solubility on the other hand is independent of pH.
Capsules of Dry Powders for Inhalation
Capsules were first used for dry powder inhalers 40 years ago with the introduction of Spinhlaer of Fison which uses two pins to puncture the capsules and deliver cromolyn sodium (sodium cromoglycate). HPMC capsules were recommended for use in unit-dose inhaler in comparison to the hard gelatin capsules, especially for hygroscopic materials. This is because the gelatin capsules have relatively high moisture content (13-16%) in comparison to 4-6% for HPMC capsules; therefore an interaction of the powdered materials with the gelatin capsule would retain much of the powder adhering to the inner surfaces of capsules resulting in much of the dose failing to leave the device. In fact one of promoting strategies for HPMC capsules is their suitability for hygroscopic materials.
Devices such as Spinhlaer, the first marketed dry powder inhaler, and Foradil inhaler (Novartis) rely on piercing the loaded capsule and withdrawing the powdered aerosol by inhalation. The holes created by piercing of the capsule were found to be different for HPMC and gelatin capsules and dependant on the relative humidity. In low humidity (below 10%) the gelatin capsule shell becomes brittle and this could cause the pierced parts of the capsule to detach, which may be inhaled causing irritation to the throat and lungs.
Capsule Coating
Enteric and colonic delivery of HPMC capsules were claimed by using coating materials of different pH solubility (at 5.5 and above and at 7 and above for enteric and colonic delivery respectively). The US patent describes how aqueous dispersions of materials such as cellulose acetate trimellitiate, hydroxypropylmethyl cellulose phthalate, polyvinyl acetate phthalate, shellac, copolymer of methacrylic acid and methylmethacrylate, azopolymers, disulphide polymers and amylase are sprayed on the filled HPMC capsules when placed in Accela-Cota 10 in order to achieve targeted delivery.
To avoid the lengthy and expensive sealing step required using the conventional capsule coating procedure and to prepare enteric-coated capsules for the use in retail or hospital pharmacy or R&D sections of pharmaceutical industry, the caps and bodies of HPMCgell capsules size 00 were coated separately prior to filling in a fluid bed apparatus, using an optimised coating process. This has resulted in effective protection of drug release from the capsules in 0.1 HCl after 2hr.
The comparison between the coating of gelatin capsules and HPMC capsules showed that the later coating was straight forward, while gelatin capsules were not suitable for direct coating when Eudragit L and S 12.5 (acrylic polymers) was used because of insufficient film adhesion to the smooth capsule surface and the brittleness of formed films. Because HPMC capsule shell surface is rougher compared to gelatin capsules as examined by scanning electron microscope, this may provide good adhesion to the coating.
IN VIVO Evaluation of the Hard Capsules
Oesophageal Sticking Tendency
Perkins and colleagues have compared the oesophageal transit of radiolabelled enteric coated tablets with similar sized and shaped gelatin capsules when administered with 50 ml of water while sitting on two separate occasions, using a population of elderly healthy volunteers (n = 23). The capsules showed tendency for longer holdups in the oesophagus (20.9 s) compared to enteric coated tablets (4.3 s).
HPMC as a bioadhesive material was reviewed and experimented by researchers. It is therefore expected that an increase in the oesophageal residence time would occur before reaching stomach when HPMC capsule is used as a result of HPMC sticking. This tendency to stick to isolated porcine oesophageal preparation was evaluated. It was found that HPMCcarr capsules detached more easily compared to gelatin capsules (P<0.001). Although their earlier findings indicated easier detachment of HPMC capsules from isolated porcine oesophagus, Honkanen et al. have recommended that both HPMC capsules as well as gelatin capsules be taken with a sufficient amount of water (150–200 ml) in an upright position and maintaining the upright position for several minutes since they found that HPMC capsules had a tendency to attach to the oesophagus. It has been shown that the in vitro porcine esophageal model is not correlated to esophageal transit in man and the recommendation was to use gamma scintigraphy to evaluate esophageal transit in man. In general, to avoid oesophagus entrapment of solid dosage forms it is advocated that they should be taken in upright body position with at least 50 mL of water to minimize entrapment in the.
The comparison of HPMCgell capsules with conventional hard gelatin capsules showed no significant differences between the two with most capsules having transit time < 20 S. In that study few of both gelatin and HPMC capsules administered had oesophageal hold-up up to 90 s when administered to eleven healthy subjects. In contrast to this, it was found that the transit times for HPMCcarr capsules and gelatin capsules of size 0, filled with lactose-based mixture, were similar and rapid ranging from 10–20 s with no prolonged oesophageal hold-ups observed with any subject. The test was carried out by administering both capsules simultaneously with 180 ml water to eight healthy male subjects following an overnight fast.
In Vivo Disintegration and Dissolution
Two prolonged release, radiolabelled formulations, containing different viscosity grades of HPMC powder (HPMC K100 and HPMC K4M) filed in HPMCcarr capsules size 0 were tested in 6 healthy volunteers with one week wash-out period between the two administrations to examine the fate of the capsules in the GIT.
The initial disintegration times for the capsules were measured as the midpoint of the time interval between the last image of the capsule with clear outlines and visually undetectable spreading of the radioactivity and the time of first detection of spreading radiation. It was found that in 4 occasions out of 12, the capsules were lodged in the oesophagus for 22–143 min. For the two formulations the initial disintegration time ranged from 33 to 75 minutes with no significant difference at the 5% level. All of the administered capsules started the disintegration in the small intestine except for two which started in the oesophagus region at 75 minutes for each of the two formulations.
In the Tuleu and colleagues study they found that all of the administered uncoated capsules HPMCcarr capsules disintegrated within 10 min in the stomach. The radiolablled capsules were filled with 550-mg dose of 4-aminosalicylic acid. A study conducted to test the disintegration of HPMCcarr and gelatin capsules in eight healthy male subjects following an overnight fast using HPMC capsules and gelatin capsules of size 0 agreed with Tuleu et al. results. Both types of capsule were first radiolabled with indium-111 and technetium-99m and then filled with a plug of lactose-based formulation. Both capsules were administered simultaneously to each individual with 180 ml of water. Initial capsule disintegration time was recorded when the scintigraphic image first shows the spread of radioactivity from the ‘core’ of the capsule. The in vivo disintegration times were not significantlydifferent (p=0.108, paired t-test) for HPMCcarr capsules (9±2 min) and gelatin capsules (7±4 min) with the first showing more consistent behavior as show in Figure 1.
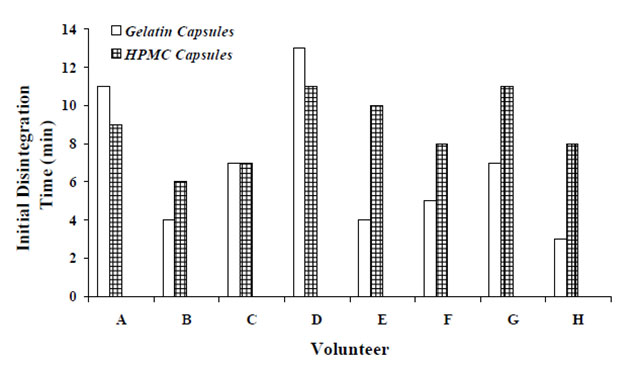
Figure 1. In vivo initial disintegration time (min) for HPMC and gelatin capsules in 8 healthy volunteers.
The studies conducted by Honkanen et al. and Tuleu et. al. were carried out on test subjects of similar characteristics in terms of age and weight and received the test capsules with 180 ml of water following an overnight fasting. Two main differences in the findings of the two studies are the lodging of HPMC capsules in the oesophagus region and the initial disintegration time. The prolonged release formulations used in Honkanen and co-workers studyand the fact that after the subjects received the capsules in sitting position were required to lie down may explain the differences observed. The formation of a gelatinous plug by the hydrated hydrophilic polymers (HPMC K100 and K4M) in water may retard the release of the formulation from the capsule shells, independent of the rupture time of the capsule shell. Not only HPMC is a bioadhesive material, but the rougher surfaces of HPMC capsules compared to hard gelatin capsules may also partially explain why HPMC capsules may lodge in the oesophagus region.
Bioavailability Studies
Tuleu and colleagues investigated using a combined scintigraphic and pharmacokinetic approach in 7 healthy volunteers, the in vivo performance of amylose–ethylcellulose-coatedHPMC capsules (size 0) as wellas uncoated capsules for the delivery of 4-aminosalicylic acid Na (550 mg) to the colon. Theresults of Tmax (29 min ± 9) and % absolutebioavailability based on AUC (118 ± 41) indicatedthat the uncoated capsule contents were released and absorbed rather completely and rapidly.
Honkanen and her colleagues found that the bioavailability of sustained release ibuprofen formulation administered orally from HPMC capsules was only significantly different from gelatin counterparts when HPMC K100 diluent was used as indicated by Tmax which occurred earlier in the case of gelatin capsules (2.19 h vs. 3.25 h, p < 0.01, n =8). Such significant differences were not observed with the higher viscosity grades of HPMC K4M and HPMC K15M. It is worth mentioning that as the use of HPMC K100 release modifier resulted in only modest sustained release effects; sharper differences between HPMC and gelatin shells would be expected for immediate release formulations. The previous results of Honkanen et al. for immediate release ibuprofen formulations did not show such differences orally, but there were differences following rectal administration. The lag time (Tlag) from HPMCcarr capsules were higher (p < 0.05, n = 8) than those for the gelatin capsules.
Four-way crossover experiment were carried out in 11 subjects in fed and fasting states by administering ibuprofen formulations filled in gelatin capsules and HPMCgell capsules. Scintigraphic and pharmacokinetics evaluations indicated that although the in vivo opening times of HPMCgell capsules were longer than gelatin counterparts, the pharmacokinetic parameters Cmax and AUC showed no significant difference. There were significant differences in the Tlag (time before absorption could be detected) for the two capsules whether in the fasted or fed state probably as a result of delayed initial disintegration of the HPMCgell capsules.
Pain has been shown to suppress nervus vagus that is responsible for gastrointestinal secretion and motility, therefore impairing the absorption of drugs administered orally resulting from reduced disintegration and dissolution. This would influence drug bioavailability from standard medications, while for rapid release formulations, dissolution and disintegration are independent of the gastrointestinal secretion and motility. It will be interesting to compare the effect of pain on drug absorption from filled HPMC and gelatin capsules, as any increase in the Tlag is clearly a disadvantageous in such condition.
IN VITRO-IN VIVO Correlation
Unlike hard gelatin capsules, HPMC capsules may have low correlation between the in vitro dissolution/disintegration and the in vivo performance. The reason for this was explained on the basis of interaction between the medium and the HPMC capsule gelling systems. It was suggested that dissolution/disintegration testing specifications should be different from that of hard gelatin capsules to reflect in vivo performance. For hard gelatin capsules, for the in vitro testing to correlate with in vivo evaluation, it has been suggested that dissolution experiment is carried out in two stages, one representing gastric medium (pepsin at pH 1.2) and the other representing the intestinal medium (pancreatin at pH 7.2). El-Malah and his colleagues indicated that the composition of the dissolution medium influences the disintegration time of the HPMC capsules, however, drug release delay in vitro may not be correlated in vivo.
While the pharmacokinetic results from in vivo oral administration studies of modified releaseibuprofen formulations indicated good agreementwith in vitro dissolution studies conducted intribasic sodium phosphate buffer of pH 7.2 (whichcontains no potassium), rectal administration didnot show such correlation. It is worthmentioning that interpatient variability inpharmacokinetic parameters was higher for rectalcompared to peroral administration.
DISCUSSION
Several HPMC capsule shells are now available and differ mainly in whether a gelling system is used or not. The gelling system is used mainly so that the manufacturing of the capsule shells can be performed using the same equipments as that of hard gelatin capsules under similar processing conditions. The gelling system used may retard the disintegration/dissolution of HPMC capsules in vitro/in vivo, but does not usually affect product bioavailability. The hindrance of in vitro disintegration/dissolution occurs when cations such as potassium (usually used in the gelling system of HPMC capsules) are present in the media, causing persistence gelling of the capsule shells. Also the in vitro disintegration/dissolution has been documented to decrease in acidic medium for HPMCgell capsules.
The use of HPMC for making capsules without a gelling system may reduce problems associated with dissolution/disintegration, however unlike hard gelatin capsules, the disintegration and dissolution of HPMC decreases as temperature increases above 30° C. Therefore there has been a suggestion to administer hard gelatin capsules with warm drink. HPMC on the other hand is soluble even below 30°C and as low as 10° C and therefore can be taken with cold water.
The machineability of the HPMC does not match that of hard gelatin capsules. Not only, the processing in the manufacture of HPMC shells may need to be altered and/or gelling system added, but also the shell may have reduced strength and much higher rejection when used in filling machines. So, dissolution/disintegration performances and the machineability of the HPMC capsules are probably the major drawback of HPMC capsules.
The main advantage of HPMC capsules over gelatin capsules could be because of their vegetable source which has wider customer acceptance. Hindu or Buddhist for example rely on vegetable sources for their nutrition. Muslim and Jews on the other hand have strict regulations about materials from animal sources and for whom vegetable source is acceptable. To this extent other types of capsules have been produced from nonanimal sources such as NPcaps capsules which are made from pullulan, a watersoluble polysaccharide produced through a fermentation process.
Fish based gelatin capsules are also available in the market. The fish gelatin solution from which the shells are produced contains mixed solution of pectin and glycerin as gelling agent and a small quantity of calcium gluconate, sucrose fatty acid esters, glacial acetic acid as additive. These capsules may offer alternative to people with concern from gelatin produced from bovine and/or porcine collagen of bones and skin.
Another reported advantage of HPMC capsules over gelatin capsules is related to the difference in moisture content of the shells. Because HPMC shells contain significantly less moisture compared to hard gelatin capsules by almost one third, it is compatible with hygroscopic materials. While HPMC shells physical strength tolerates wide range of environmental conditions, hard gelatin capsules readily becomes brittle and unusable in low humidity. One offered solution to this problem is to add PEG 4000 to the gelatin. As such the brittleness of the capsules will be minimized and encapsulation of hygroscopic materials becomes possible. Ciper and Bodmeier have found that the addition of PEGs (400 or 1500 but not 4000) up to 5% w/w to the gelatin resulted in a significant disintegration time decrease in vitro (44± 6 s) and in the mouth of four healthy volunteers (13 ± 4 s) without affecting the mechanical properties of the capsules. Similar results were obtained when xylitol and sorbitol were used instead of PEGs. It is possible that the incompatibility of PEG and gelatin is why such capsules have not been produced in large scale.
The cross-linking of gelatin that affects product dissolution and disintegration has not been observed for HPMC capsules under similar conditions. This makes HPMC capsules compatible with wider range of products except for some oxidizing agent.
It may be expensive for the pharmaceutical industry to reformulate their products to make use of HPMC capsules as the benefits achieved might not be weighing out the cost. However, for new capsule products, HPMC capsules should become an option. Currently marketed products using HPMC capsules filled with herbal formulations benefit from flexible regulations over this category of supplements. Such regulations are expected to be tougher in the future which may lead to the number of HPMC capsule products to become static. In order to expand the use of HPMC capsules in new products, official bodies such as FDA should endorse their use and pharmacopeias should start to provide monographs regarding the specification and tests carried out for such capsules. With more interest exercised from the big pharmaceutical manufacturers to use HPMC capsules, definitely there will be greater number of products in the market.
CONCLUSION
Two important areas where improvements have to be achieved in order to qualify the HPMC capsules ahead of gelatin capsules are in their machineability and in the in vitro and in vivo disintegration/dissolution performances. The main area where HPMC capsules can have better prospect compared to gelatin capsules is in wider patients’ preferences and the dietary sensitivities in certain markets.
|
 沪公网安备 31011202012935号 Power by CN7080
沪公网安备 31011202012935号 Power by CN7080